PAUL HAY Capital Projects
Pipe Size Calculation
Author: Paul Hay
e-mail: paul.hay@phcjam.com
profile: www.linkedin.com/in/phcjam
Problem:- Find service pipe size given the following:
Street main pressure [E] = 350 kPa
Height of uppermost fixture
(above street) = 10 m
Uppermost fixture = WC with flush valve
Total fixture units in the systema = 85
Total fixture units in the systema = 85
a. See table 1 for supply fixture units of individual plumbing fixtures.
Developed length (DL) of piping (to
highest and most remote fixture) = 30 m
Predominant flushing mechanism = Flush valves
Solution:- Calculation of Service Pipe Size
1. Find the pressure required in the system to provide the minimum fixture pressure [A] for uppermost fixtureb:
A = 103 kPa
Table 2: Minimum Pressure & Flow required for typical plumbing fixtures [source:- Mechanical & Electrical Equipment in Buildings]
2. Calculate the Static Head [B]:
B = 10 kPa/m x Height of uppermost fixture
= 10 x 10 m
= 100 kPa
Table 3: Minimum pipe sizes for typical plumbing fixtures [Source:- National Building Code of Jamaica 1983]
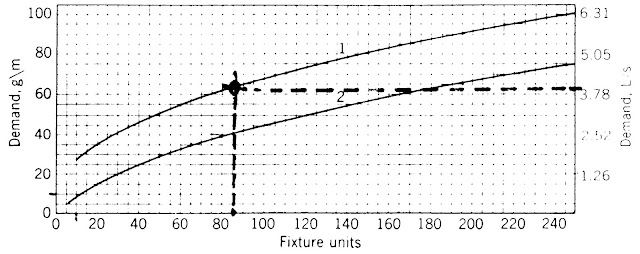
Figure 1: Demand Load for predominant (1) flush valves & (2) flush tanks [source:- Mechanical & Electrical Equipment in Buildings]
3. Using total fixture units, determine the demand loadc for the relevant flushing mechanism from figure 1.
5. Using demand load [3] and approx. pipe size [4], determine the pressure loss in the water meter [D] from fig. 3:
D = 62 kPa
6. Calculate the maximum frictional loss [C] that can be tolerated in the service pipe:
C = E - (A + B+ D)
= 350 - (103 + 100 + 62) = 350 - 265
= 85 kPa
7. Calculate the Pipe length equivalent of fittings
[DL'] (estimated at 20 % of DL):
DL' = 0.2 x DL = 6 m
8. Calculate the total equivalent length (TEL) of the piping:
TEL = DL + DL' = 30 + 6 = 36 m
9. Calculate the unit-frictional loss of the pipe:
100 x C/TEL = 100 x 85/36 = 189 kPa
e. 50 mm dia. service pipe size